Limit Testing
Test component substance to defined limit
What is Limit Testing?
Although it is widely known that pharmacopoeial Purified water and water for injection are essential in the pharmaceutical manufacturing setting, other grades of water require testing to client-specific limits. Limit testing is a very general term that refers to the testing of any component substance to a defined limit – usually required on a case-by-case basis that are niche to one client.
Why do we do it?
A limit test may be required with a specific industry regulatory requirement. Pharmaceutically it is a requirement to perform Microbiological and Chemical testing on Purified Water and Water for Injectin (WFI). In addition to regulatory requirements, specific clients may need to demonstrate control of a particular substance component for reasons specific to their process.
How can we help assure compliance?
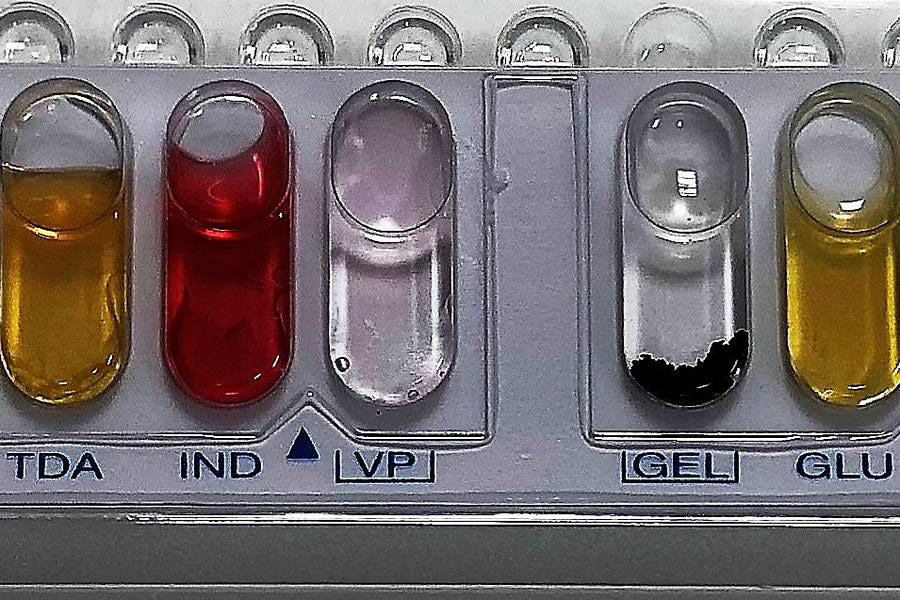
At Honeyman we have a broad range of experience with limit testing on a wide variety of sample presentations. Our cGMP chemistry limit testing is often carried out using colourimetric testing. More specialised tests can be performed by volumetric titration techniques upon request. HPLC SECTION REMOVED** Our cGMP microbiological limit testing is largely performed via membrane filtration total viable count. Some examples of the limit testing we can provide include:
- Membrane filtration TVC
- TOC
- Calcium and Magnesium
- Chloride
- Arsenic
- Iron
- Heavy Metals
- Sulphate
- Potassium Permanganate
- Oxidisable Substances
- Total dissolved solids
- Manganese
- Silicates
- Phosphate
- Ammonium
This is list is not exclusive, please enquire now to see if we can help with your particular requirement.
Our Customers: